THE INTERNATIONAL WORKSHOP ON MEIBOMIAN GLAND DYSFUNCTION: EXECUTIVE SUMMARY
Meibomian gland dysfunction (MGD) may well be the leading cause of dry eye disease throughout the world. Although this condition affects the health and well-being of millions of people, there is no global consensus on the definition, classification, diagnosis, or therapy for MGD. To achieve such a consensus, the Tear Film and Ocular Surface Society (TFOS; http://www.tearfilm.org), a nonprofit organization, launched the International Workshop on Meibomian Gland Dysfunction (www.tearfilm.org/mgdworkshop/index.html). The objectives of the workshop were to:
- conduct an evidence-based evaluation of meibomian gland structure and function in health and disease;
- develop a contemporary understanding of the definition and classification of MGD;
- assess methods of diagnosis, evaluation, and grading of the severity of MGD;
- develop recommendations for the management and therapy of MGD;
- develop appropriate norms of clinical trial design to evaluate pharmaceutical interventions for the treatment of MGD; and
- create a summary of recommendations for future research in MGD.
The report of the Workshop on MGD, which required more than 2 years to complete, was finalized in 2010. This effort involved more than 50 leading clinical and basic research experts from around the world. These participants, who were assigned to subcommittees, reviewed published data and examined the levels of supporting evidence. Subcommittee reports were circulated among all workshop participants, presented in open forum, and discussed in an interactive manner.
The entire workshop report is published in English in this issue of IOVS. The report has also been translated, at least in part, into Chinese, Dutch, French, German, Greek, Italian, Japanese, Polish, Portuguese, Spanish, Russian and Turkish; these translations are available on the TFOS website.
An executive summary of the conclusions and recommendations of the TFOS Workshop on MGD is presented in this article. The material is abstracted from the full report, and thus, additional details and references can be obtained in the open-access, online version.
Definition and Classification of MGD
Meibomian gland dysfunction (MGD) is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.
There are several evidence-based explanations for the terminology used in this definition. The term dysfunction is used because the function of the meibomian glands is disturbed. The term diffuse is used because the disorder involves most of the meibomian glands. Localized involvement of meibomian glands, such as in chalazion, tends not to cause abnormalities in the tear film or ocular surface epithelia and therefore is not considered to belong within the context of MGD. Obstruction of the meibomian gland orifices and terminal ducts and qualitative and/or quantitative changes in meibomian gland secretions are identified as the most prominent aspects of MGD. In addition, subjective symptoms of eye irritation are included in the definition, as it is the symptoms that are of greatest concern to the patient and often to the clinician. Improvement in the patient's symptoms is the major goal in the treatment of MGD. The role of inflammation in the etiology of MGD is controversial and uncertain.
Recent literature has used the terms posterior blepharitis and MGD as if they were synonymous, but these terms are not interchangeable. Posterior blepharitis describes inflammatory conditions of the posterior lid margin, of which MGD is only one possible cause. In its earliest stages, MGD may not be associated with clinical signs characteristic of posterior blepharitis. At this stage, affected individuals may be symptomatic, but alternatively, they may be asymptomatic and the condition regarded as subclinical. As MGD progresses, symptoms develop and lid margin signs, such as changes in meibum expressibility and quality and lid margin redness, may become more visible. At this point, an MGD-related posterior blepharitis is said to be present.
The term MGD is regarded as appropriate for describing the functional abnormalities of the meibomian glands. Meibomian gland disease is used to describe a broader range of meibomian gland disorders, including neoplasia and congenital disease. Other terms such as meibomitis or meibomianitis describe a subset of disorders of MGD associated with inflammation of the meibomian glands. Although inflammation may be important in the classification and in the therapy of MGD, these terms are not sufficiently general, as inflammation is not always present.
MGD may be classified according to anatomic changes, pathophysiological changes, or the severity of disease. Any classification system must meet the needs of the clinician and researcher alike. A classification based on pathophysiology is deemed to best meet these needs.
A classification of MGD into two major categories based on meibomian gland secretion is proposed: low-delivery states and high-delivery states (Fig. 1). Low-delivery states are further classified as hyposecretory or obstructive, with cicatricial and noncicatricial subcategories. Hyposecretory MGD describes the condition of decreased meibum delivery due to abnormalities in meibomian glands without remarkable obstruction. Obstructive MGD is due to terminal duct obstruction. In the cicatricial form, the duct orifices are dragged posteriorly into the mucosa, whereas these orifices remain in their normal positions in noncicatricial MGD. High-delivery, hypersecretory MGD is characterized by the release of a large volume of lipid at the lid margin that becomes visible on application of pressure onto the tarsus during examination. Each MGD category also has primary causes, referring to conditions for which there are no discernible underlying causes or etiology.
Overall, MGD can lead to alterations of the tear film, symptoms of eye irritation, inflammation, and dry eye.
Anatomy, Physiology, and Pathophysiology of MGD
The meibomian glands are large sebaceous glands located in the tarsal plates of the eyelids. These glands actively synthesize and secrete lipids and proteins that are delivered at the upper and lower eyelid margins just anterior to the mucocutaneous junctions. The glandular lipids spread onto the tear film, promote its stability, and prevent its evaporation.
Meibomian glands, unlike other sebaceous glands, do not have direct contact with hair follicles. Each meibomian gland consists of multiple secretory acini-containing meibocytes, lateral ductules, a central duct, and a terminal excretory duct that opens at the posterior lid margin. The number and volume of meibomian glands is greater in the upper than in the lower lid, but the relative functional contribution of the upper and lower lid glands to the tear film remains to be determined. Also unknown is the source or sources of stem cells for this gland.
Meibomian glands are densely innervated, and their function is regulated by androgens, estrogens, progestins, retinoic acid, and growth factors, and possibly by neurotransmitters. The glands produce polar and nonpolar lipids through a complex and incompletely understood process. These lipids are secreted into the ducts through a holocrine process. Meibum delivery onto the lid margin occurs with muscular contraction during lid movement.
Meibomian gland dysfunction is caused primarily by terminal duct obstruction with thickened opaque meibum containing keratinized cell material. The obstruction, in turn, is due to hyperkeratinization of the ductal epithelium and increased meibum viscosity (Fig. 2). The obstructive process is influenced by endogenous factors, such as age, sex, and hormonal disturbances, as well as by exogenous factors such as topical medication. The obstruction may lead to intraglandular cystic dilatation, meibocyte atrophy, gland dropout, and low secretion, effects that do not typically involve inflammatory cells. The outcome of MGD is a reduced availability of meibum to the lid margin and tear film. The consequence of insufficient lipids may be increased evaporation, hyperosmolarity and instability of the tear film, increased bacterial growth on the lid margin, evaporative dry eye, and ocular surface inflammation and damage.
Overall, MGD is an extremely important condition, conceivably underestimated, and very likely the most frequent cause of dry eye disease.
Tear Film Lipids and Lipid–Protein Interactions in Health and Disease
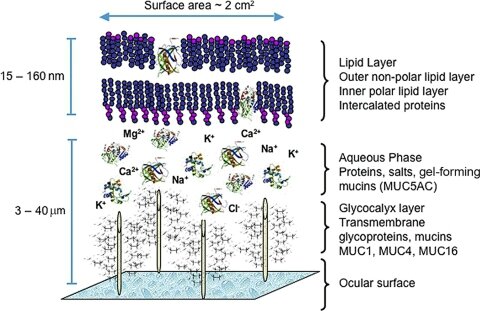
The meibomian glands are the main source of lipids for the human tear film. The meibomian gland secretions consist of a complex mixture of various polar and nonpolar lipids containing cholesterol and wax esters, diesters, triacylglycerol, free cholesterol, free fatty acids, and phospholipids. The meibum spreads onto the tear film and functions to slow evaporation of the aqueous component, preserve a clear optical surface, and form a barrier to protect the eye from microbial agents and organic matter such as dust and pollen.
A proposed model of the human tear film is shown in Figure 3. This model incorporates proteins (e.g., lipocalin, lysozyme, and the surfactant proteins B and C) intercalated in and/or adsorbed to the outer lipid layer. These protein interactions appear to influence the physical properties and surface tension of the tear film lipid layer. The proposed model also features very long chain (O-acyl)-ω-hydroxy fatty acids, which may act in the formation of an intermediate surfactant lipid sublayer between the outermost nonpolar lipids and the aqueous layer of the tear film.
The lipid patterns of human meibum show many similarities among normal individuals, but may differ from those in persons with MGD. Some of these differences may be due to an increased presence of certain types of commensal lid bacteria that can hydrolyze lipids. Indeed, the ability of antibiotics to inhibit bacterial lipolytic enzymes may in part explain the effectiveness of such pharmaceuticals in the treatment of MGD.
Lipid profiles in human meibum differ from those in the tear film. Of particular interest, the absolute and relative amounts of polar lipids in both the meibum and the tear film have yet to be resolved.
Another attribute of tear film lipids is that they appear to be essential for ease and comfort in contact lens wear, but also form deposits on these lenses. It is possible that contact lens wear itself disrupts the meibomian glands and/or lipid layer and leads to tear film evaporation and ocular surface discomfort.
Epidemiology and Associated Risk Factors for MGD
Although the etiology of MGD may differ from that of aqueous-deficient dry eye disease (which is due to insufficient lacrimal gland production), the two conditions share many clinical features, including symptoms of ocular surface irritation and visual fluctuation, altered tear film stability, and potential ocular surface compromise. When the severity of MGD is of a sufficient degree, it may give rise to the second major subtype of dry eye disease, evaporative dry eye. These subtypes are not mutually exclusive.
Epidemiologic investigation of MGD has been limited because there is no consensus regarding the definition nor is there a standardized clinical assessment that characterizes this disease. There is a paucity of evidence on the natural history of MGD, the actual processes that cause it, or when symptoms actually develop in the disease process. It is also unclear whether MGD symptoms begin at the onset of or after meibomian gland damage and altered meibum delivery or instead arise from subsequent damage to other ocular surface tissues.
The reported prevalence of MGD varies widely. A striking observation is that the prevalence of MGD appears to be much higher in Asian populations (Table 1), often reported as greater than 60% in different Asian population-based studies. In contrast, the prevalence in Caucasians spans from 3.5% to 19.9%. Many people with the clinical signs of MGD also have overlapping symptoms of dry eye disease.
Several ophthalmic, systemic, and medication-related factors may coexist with, or plausibly contribute to, the pathogenesis of MGD. Ophthalmic factors may include anterior blepharitis, contact lens wear, Demodex folliculorum, and dry eye disease. Systemic factors that may promote MGD include, among others, androgen deficiency, menopause, aging, Sjögren's syndrome, cholesterol levels, psoriasis, atopy, rosacea, hypertension, and benign prostatic hyperplasia (BPH). Medications associated with the pathogenesis of MGD include antiandrogens, medications used to treat BPH, postmenopausal hormone therapy (e.g., estrogens and progestins), antihistamines, antidepressants, and retinoids. The ω-3 fatty acids may be protective.
In summary, MGD appears to be a prevalent problem, with detriments that are potentially damaging to well-being. Nonetheless, even basic information regarding its prevalence, demographic and geographic distributions, risk factors, and impact on ocular health and quality of life are only beginning to emerge. The same was said of dry eye disease more than a decade ago, and since that time, research efforts have grown exponentially. We are confident that the time has now come to embark on the systematic study of MGD as well. It is through such efforts that a better understanding of the disease will be gained, and strategies for prevention and treatment will begin to be developed.
Diagnosis of MGD
The diagnosis of MGD, whether in isolation or associated with ocular surface damage or dry eye, should be viewed in the context of diagnosing any ocular surface disease. Tests should be performed in an order that minimizes the extent to which one test influences the results of the tests that follow. A series of tests that are recommended for use in the diagnosis of MGD and in MGD-related disorders, including evaporative dry eye, is presented in Table 2.
In asymptomatic adults, it is appropriate to include gland expression (e.g., by the application of moderate digital pressure to the central lower lid) to the routine workup of the patient, to detect asymptomatic, nonobvious MGD. A diagnosis of MGD may require that the patient be further assessed for ocular surface damage and dry eye, using appropriate diagnostic techniques.
In patients with ocular surface symptoms or morphologic lid signs of MGD (e.g., orifice plugging and other orifice or lid margin signs), meibomian gland functionality should be assessed by digital pressure over the central (± nasal) third of the lower and upper lids, to determine the extent and severity of the MGD (expressibility and secretion quality). The examination should be performed with moderate digital pressure or by a standardized technique. The patient should be further assessed for evidence of ocular surface damage and dry eye.
A two-tiered approach to the diagnosis of MGD-related dry eye is recommended. In the first step, normal subjects are distinguished from patients with dry eye of any type (generic dry eye). The second step involves the differential diagnosis of MGD-related evaporative dry eye and aqueous-deficient dry eye.
Two approaches are proposed: one suitable for practitioners working in a general clinic and the other for investigators working in specialized units. The evidence base of the tests proposed varies according to the clinical setting.
A suitable sequence of tests to perform in a general clinic for the diagnosis of MGD-related disease in patients presenting with symptoms of ocular surface disease is as follows:
- Administration of a symptom questionnaire;
- Measurement of the blink rate and calculation the blink interval;
- Measurement of lower tear meniscus height;
- Measurement of tear osmolarity (if available);
- Instillation of fluorescein and measurement of the tear film breakup time (TFBUT) and Ocular Protection Index (OPI);
- Grading of corneal and conjunctival fluorescein staining;
-
Schirmer test or alternate (phenol red thread test).Positive (abnormal) results in tests 1, 4, 5, and 6 provide partial evidence of the presence of a generic dry eye, without specifying whether it is aqueous-deficient or evaporative. Evidence of aqueous-deficient dry eye may be obtained by measuring tear flow or an assessment of aqueous volume on the basis of tear meniscus height or Schirmer test.
- If MGD has not been characterized (symptomatic/asymptomatic) at a previous visit, then it can be assessed at the end of this sequence as follows:
- Quantification of morphologic lid features
- Expression: quantification of meibum expressibility and quality
- Meibography: quantification of dropout.
If testing suggests the diagnosis of a generic dry eye and tests of tear flow and volume are normal, then an evaporative dry eye is implied and quantification of MGD will indicate the meibomian gland contribution. This test sequence also permits a diagnosis of symptomatic MGD to be made, with or without ocular surface staining and with or without dry eye. The graded scores for each test can be used to monitor the disease during treatment.
An “ideal” or comprehensive test series for corneal specialists or for investigators engaged in clinical trials is also proposed for clinics that have access to a wider range of diagnostic equipment. Some of the tests listed are alternatives and are more research based. It is suggested again that the diagnosis be performed in two steps: first to diagnose generic dry eye, and then to subtype with the grade of MGD.
This test series consists of a symptom assessment (e.g., the Ocular Surface Disease Index [OSDI] and the Dry Eye Questionnaire [DEQ]) and measurements of the osmolarity, secretion, volume, stability, and evaporation of tears. Tests of ocular surface damage, such as corneal and conjunctival staining, are also included in the diagnostic series. The results of tests of inflammatory mediators, the presence of inflammatory cell markers, and other proteomic and lipidomic mass spectrometry analyses can also be assessed to provide information regarding overall ocular surface inflammatory status, although the link to MGD specifically is not known at this time. Specific measures of tear production for the diagnosis of aqueous-deficient dry eye are also recommended.
Management and Therapy of MGD
Treatment of MGD varies greatly among eye care providers on different continents. Underreporting makes it difficult to assess practice patterns accurately, but most practitioners agree that underdiagnosis is common and clinical follow-up irregular.
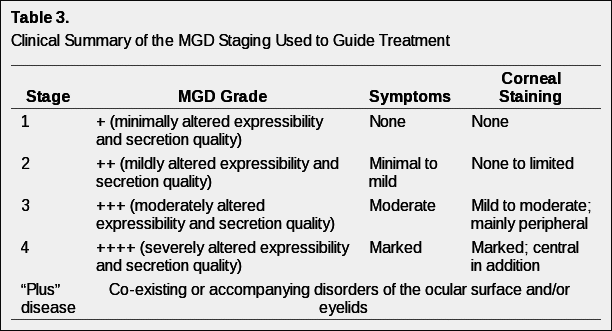
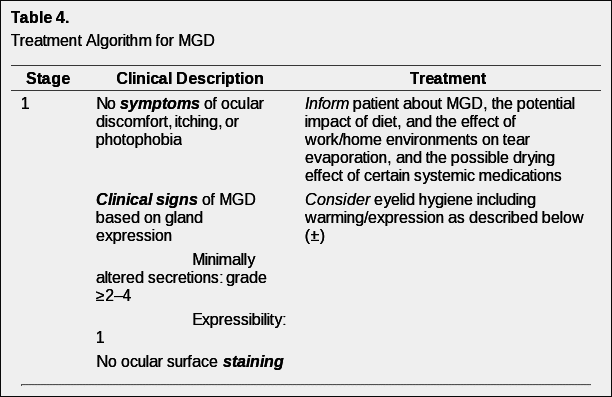
Without generally accepted definitions for a staging system of clinical severity of MGD, it is problematic to propose a treatment plan based on disease stage. Nonetheless, in the hope of assisting eye care providers attempting to fashion a logical, evidence-based treatment approach, a disease-staging summary (Table 3) and staged treatment algorithm (Table 4) are proposed.
In the staging of disease, it is recognized that it is difficult clinically to separate the effects of MGD and the effects of aqueous deficiency on the ocular surface. In addition, co-morbid diseases are often present. Thus, Table 3 represents a clinical picture of staged disease. Co-morbid conditions, defined as plus disease may require concurrent management according to standard-of-care protocols.
Table 4 reflects an evidence-based approach to the management of MGD. At each treatment level, lack of response to therapy moves treatment to the next level. A ± sign means that the evidence to support the use of the treatment at that level is limited or emerging, thus its use should be based on clinical judgment. A + sign indicates that the treatment is supported by the evidence at that stage of disease. The quality of expressed meibum and meibum expressibility are key features in the clinical assessment of MGD.
As outlined in Table 4, meibum quality is assessed in each of eight glands of the central third of the lower lid, and meibum expressibility is assessed in the five glands in the lower or upper lid. The numerical staining scores refer to a summed score of staining of the exposed cornea and conjunctiva. Note that corneal staining with topically instilled fluorescein can occur in normal subjects on a sporadic basis, therefore pathologic staining should be identified as repeatedly observed staining of the same or adjacent portions of the cornea.
With every systemic medication, systemic side effects have to be considered. With the treatment algorithm in Table 4 in mind, the phototoxicity caused by systemic tetracycline derivatives and the anticoagulant effects of essential fatty acids (EFAs) are of specific concern. EFAs are nutritional supplements that have received much attention, but with only one published clinical study so far supporting their efficacy in MGD. This is also true of the use of sex hormones, for which there is no published clinical trial regarding efficacy, and there is no licensed product available. Hence, the panel agreed not to assign this potential treatment modality to a grade of disease. The risks of prolonged topical corticosteroid therapy (e.g., induction of cataract and elevated intraocular pressure) are well known. Consequently, the use of such medications should be reserved for the treatment of acute exacerbations in MGD and are not recommended for long-term therapy. Regular monitoring of intraocular pressure is mandatory with the use of topical corticosteroids.
Management of plus disease conditions should follow the standard of care and is not limited to the treatments listed in Table 4.
Clinical Trials
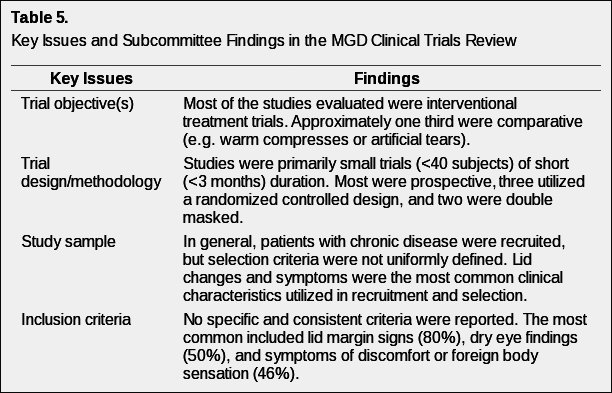
There are significant limitations in assessing the available literature regarding clinical trial methodology in MGD. The lack of consensus in terminology and the broad array of clinical tests performed in clinical trials involving the meibomian gland and eyelid create a discordance in comparing results across studies.
Table 5 provides an overview of the topical areas in clinical trials (objectives, design, sample, inclusion, exclusion, outcomes, treatments, and statistical design) that were reviewed in the 26 papers identified as clinical trials involving MGD.
A recommendation for the design of clinical trials specific to MGD is to include well-defined objectives. These objectives should be clearly stated and allow for concise and specific questions to be answered. There are important and basic questions and considerations to address in MGD clinical trial design:
- Studies must be designed to distinguish between MGD and dry eye disease. A review of past clinical trials of MGD suggested that there is no clear consensus on what such studies should entail. Some include subjects with dry eye, others exclude them, and still others fail to consider dry eye status altogether. Studies that evaluate the possible role of MGD in aqueous-deficient dry eye and the overlap of the two would also be welcome.
- Given that there is considerable uncertainty between MGD and dry eye disease, trials that evaluate the association between the two would be beneficial, as would observational trials that assess the natural history of MGD. Of special value would be a standardized symptom questionnaire that could distinguish MGD lid disease from dry eye disease.
- Developing alternative or indirect ways of assessing and testing MGD would also be desirable. Accurate, repeatable measures of symptoms are of obvious value as outcome measures and are directly relevant to the patient's health. Quantitative measures of disease may also be useful, especially if it can be shown that reversal improves long-term health. Examples include osmolarity, interferometry, high resolution OCT, tests that can measure visual function and interblink visual acuity decay, and techniques that discriminate differences in the meibum. It is important that clinical studies that demonstrate the correlation between the results of these tests and clinical findings, such as symptoms and signs, be conducted first.
Overall, the most desirable clinical trials for the evaluation of MGD treatments are prospective, randomized, controlled, and double-masked. To date, very few trials have met these criteria, and it is unknown when, if ever, results from those ongoing trials will be published.
We suggest the following main priorities in future clinical trials in MGD:
- Determine the natural history of MGD;
- Clarify the association between MGD and dry eye disease;
- Develop a specific and validated questionnaire for symptoms of MGD;
- Create a standardized grading for lid and other signs in MGD;
- Evaluate the feasibility and clinical value of lipid and protein biomarkers;
- Validate surrogate clinical outcomes related to MGD.
Acknowledgments
The authors thank Michelle Dalton (www.dalton-and-associates.com) for her professional assistance with this Workshop highlight report.
Footnotes
Supported by the Tear Film and Ocular Surface Society (TFOS; http://www.tearfilm.org); individual author support is listed in the Appendix of the Introduction.
Disclosure: Each Workshop Participant's disclosure data can be found in the Appendix of the Introduction.

 1
1 




/http%3A%2F%2Fstorage.canalblog.com%2F52%2F98%2F1309572%2F106438658_o.gif)
/http%3A%2F%2Fstorage.canalblog.com%2F56%2F72%2F1309572%2F106274822_o.jpg)
/http%3A%2F%2Fstorage.canalblog.com%2F03%2F08%2F1309572%2F105683615_o.jpg)
/http%3A%2F%2Fstorage.canalblog.com%2F22%2F13%2F1309572%2F105656506_o.jpg)